Stanford Medicine begins enrolling for COVID-19 vaccine trial. Ad See required Emergency Use Authorization EUA and safety information.

Parents Will You Vaccinate Your Children For Covid The New York Times
In the fight against COVID-19 a vaccine is a critical part of addressing the global health crisis by decreasing rates of infection disease and death worldwide.
How to enroll in covid vaccine trial. Find information about the vaccine intended for vaccination providers. The main objective for every clinical trial is safety Muller says. Hospitals universities and private research sites offer clinical trials for drugs or to evaluate medical conditions.
November 5 2021. Even in those trials that are in relatively late stages such as the COVID vaccine trials. On NPRs Planet Money Dr.
People who have not yet received an FDA authorized COVID-19 vaccine are also eligible to enroll in the trial in a separate cohort. Del Rio said he enrolls six or seven study subjects a week in a typical clinical trial but for the Covid vaccine trial hell try to enroll that number in a day. Collins reflects on efforts to enroll diverse participants in the COVID-19 vaccine trials.
Johnson Johnson is one of many companies hard at work on an investigational COVID-19 vaccine candidate which includes recruiting up to 60000 adult participants 18 and older and of all genders and racial and ethnic groups around the world for a Phase 3 clinical trial. Initially these volunteers will receive the two-dose Moderna COVID-19 vaccine regimen and will be assigned to receive a booster dose of a vaccine. To be a vaccination provider your health system or you as an independent provider are required to sign the CDC COVID-19 Vaccination Program Provider Agreement.
Research in the form of clinical trials. The drug maker said it will give the. Collins is featured in an NPR Planet Money episode on the story behind Operation Warp Speed and the development of COVID-19 vaccines.
How to enroll in a clinical trial. The Phase 3 clinical trial was designed to determine if the Pfizer-BioNTech COVID-19 vaccine is safe and effective in preventing COVID-19 disease. And Mexico to test whether the investigational vaccine candidate NVX-CoV2373 will prevent COVID-19 disease.
More than 100 clinical trial sites at hospitals and medical clinics across the world will be testing potential COVID-19 vaccines. It will compare this investigational vaccine. On November 18 Pfizer and BioNTech announced that after conducting the primary efficacy analysis their mRNA-based COVID-19 vaccine.
The PVTU working with UPMC Childrens Hospital of Pittsburgh is one of 100 study sites in the US. Participate in Testing Treatment Vaccine Research. The Pittsburgh Vaccine Trials Unit PVTU has joined the pediatric COVID-19 Moderna KidCOVE vaccine trial and is enrolling children ages 6 months to 12 years.
Eventually he aims to have a. Stanford plans to enroll about 1000 people as part of a large Phase 3 trial to determine whether a vaccine can protect against infection with the coronavirus. You must be legally authorized in your jurisdiction to administer vaccines.
Studying the COVID-19 Vaccine For Children. Tests vaccines and treatments will help slow and possibly put an end to COVID-19 and the impact its had on the world and in our communities. COVID-19 vaccines in trials do not give recipients the virus.
Modernas pediatric covid-19 vaccine trial is called Kidcove. The landmark Pfizer-BioNTech Phase 3 clinical trial. And Canada participating in the study which seeks to enroll.
The drug maker said it will give the vaccination to 6750 children in the US and Canada. Things to know before signing the agreement. This trial began July 27 2020 and completed enrollment of 46331 participants in January 2021.
Kentuckys COVID vaccine trials. The PREVENT-19 PRE-fusion protein subunit Vaccine Efficacy Novavax Trial COVID-19 clinical trial will enroll up to 30000 adults in approximately 115 locations in the US. Is It Safe to Enroll Your Kid in a COVID Vaccine Trial.
What Are The Long Term Side Effects Of Covid Vaccines 3 Things To Know The Reporter Uab

Moderna Trial Studies Covid 19 Vaccine For Children Uchealth Today

Cdc Vaccine Group Don T Delay Second Doses Of Covid 19 Vaccine American Academy Of Pediatrics

When Will A Covid 19 Vaccine Be Ready For Kids Under 12 And What S The Latest News On Clinical Trials Connecticut Children S
Covid Vaccines For Kids Under 12 Expected Midwinter Fda Official Says

Kids Covid Vaccine Side Effects What To Know

Fda Oks Pfizer Covid 19 Vaccine For 12 15 Age Group Coronavirus Updates Npr
Covid Vaccine Pfizer Begins Trial On Infants And Young Kids

Covaxin India S Homegrown Covid Vaccine Brings Hope And Controversy
Covid Booster Trial Will Give Third Vaccine Dose To Uk Volunteers Medical Research The Guardian

Understanding Covid 19 Vaccine Trials In Children Cedars Sinai

Age And Frailty In Covid 19 Vaccine Development The Lancet
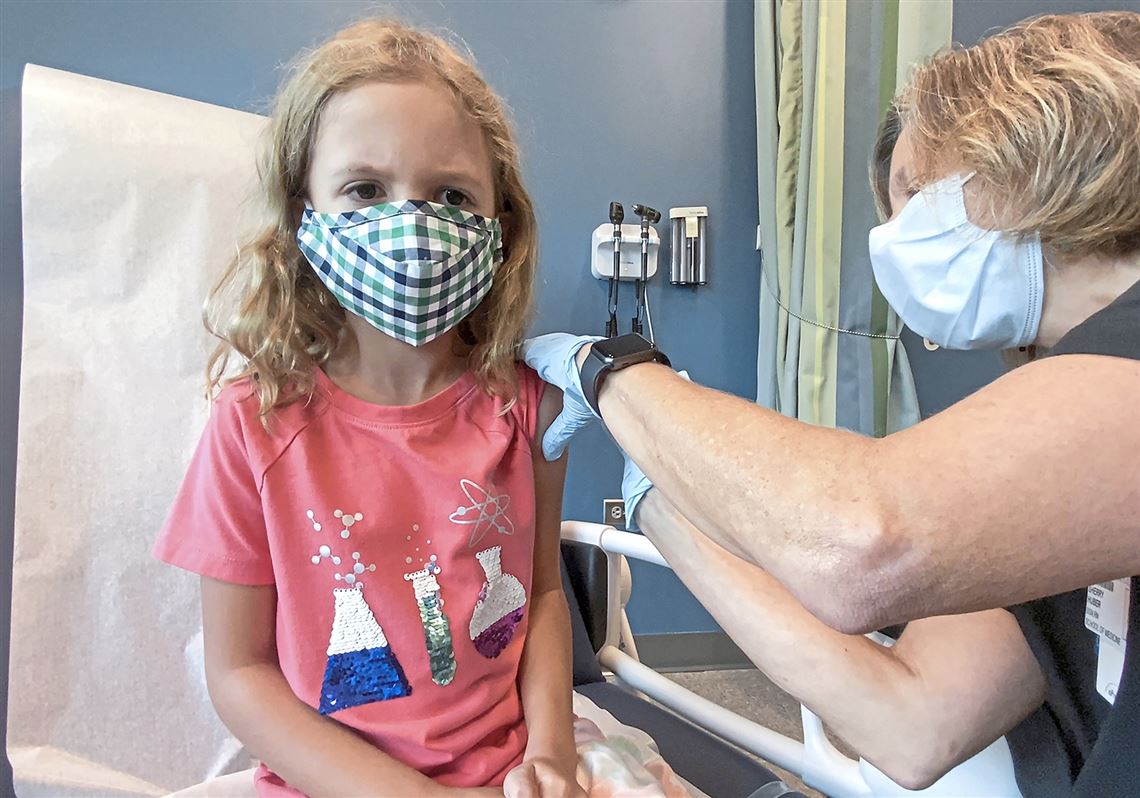
What To Know About Young Kids And The Covid 19 Vaccine Pittsburgh Post Gazette

Learn More About The Phase 3 Studies Of Our Investigational Covid 19 Vaccine Candidate And Our Commitment To Safety And Transparency Johnson Johnson

Covid Vaccine Pfizer Begins Trial On Infants And Young Kids
Coronavirus Vaccine Timeline What To Watch Leading Research
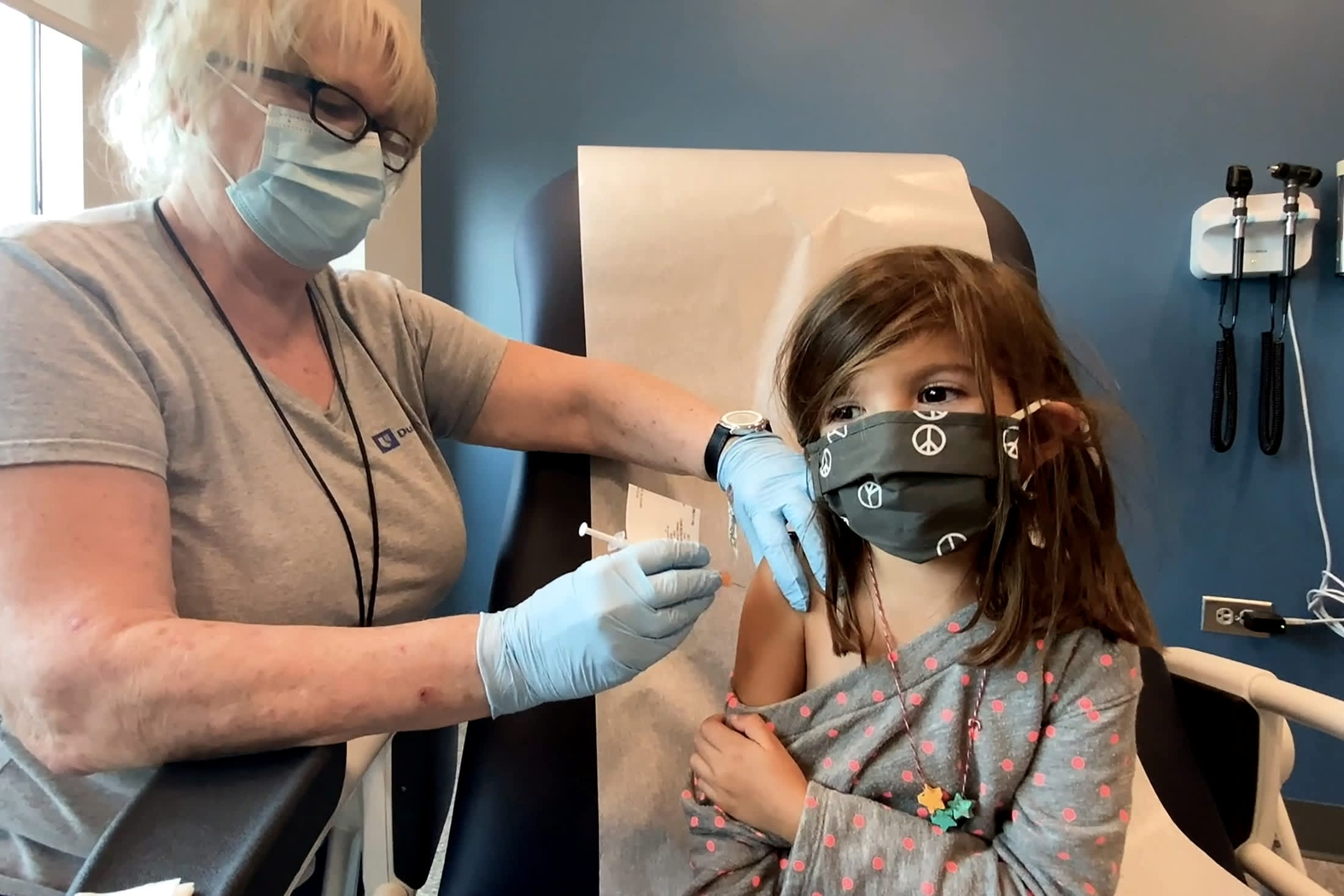
Updates Of Fda Meeting On Pfizer S Covid Vaccines For Kids Ages 5 To 11
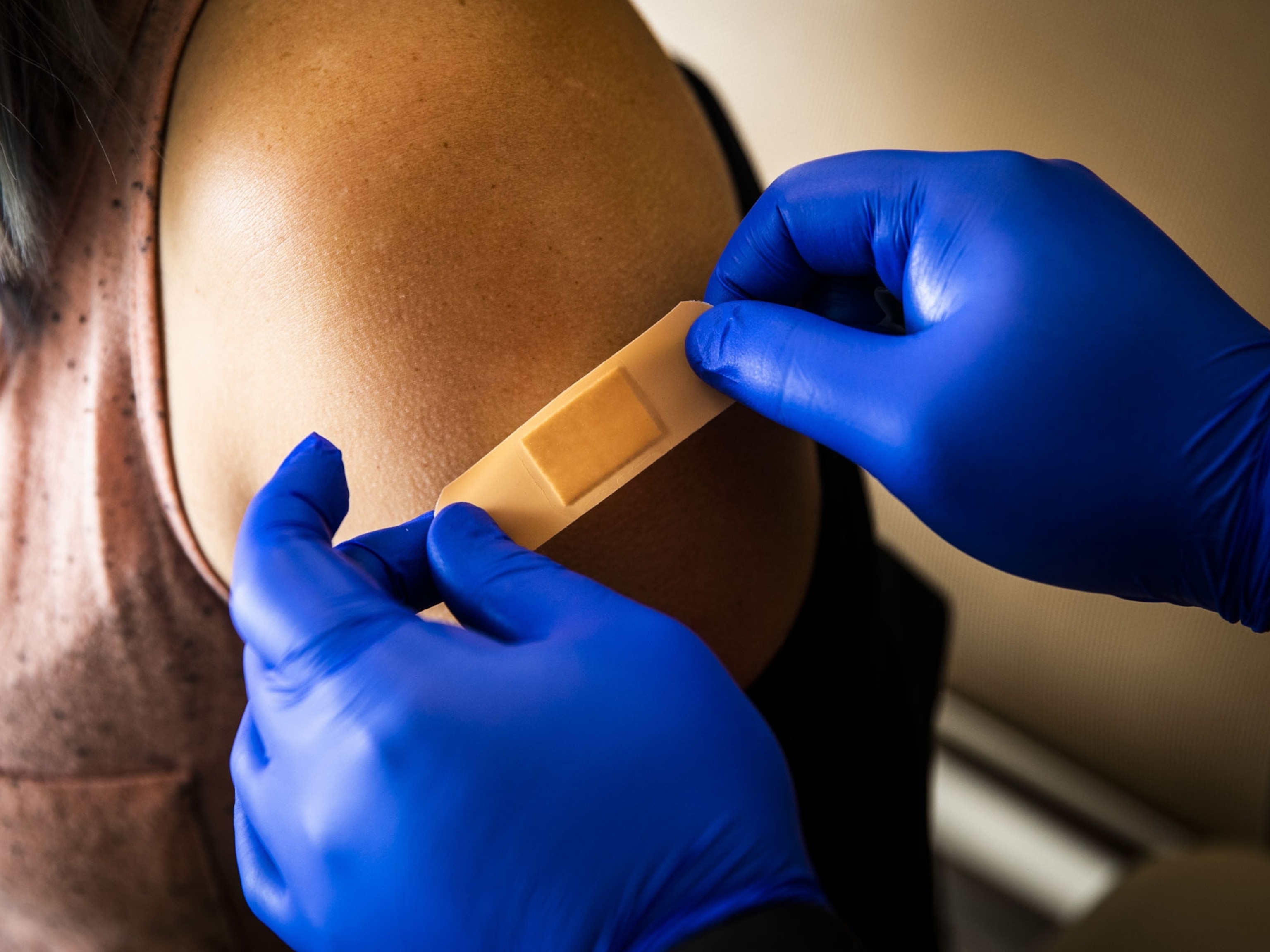
Here S The Latest On Covid 19 Vaccines
Drug Companies Recruit Black Americans For Covid 19 Vaccine Trials
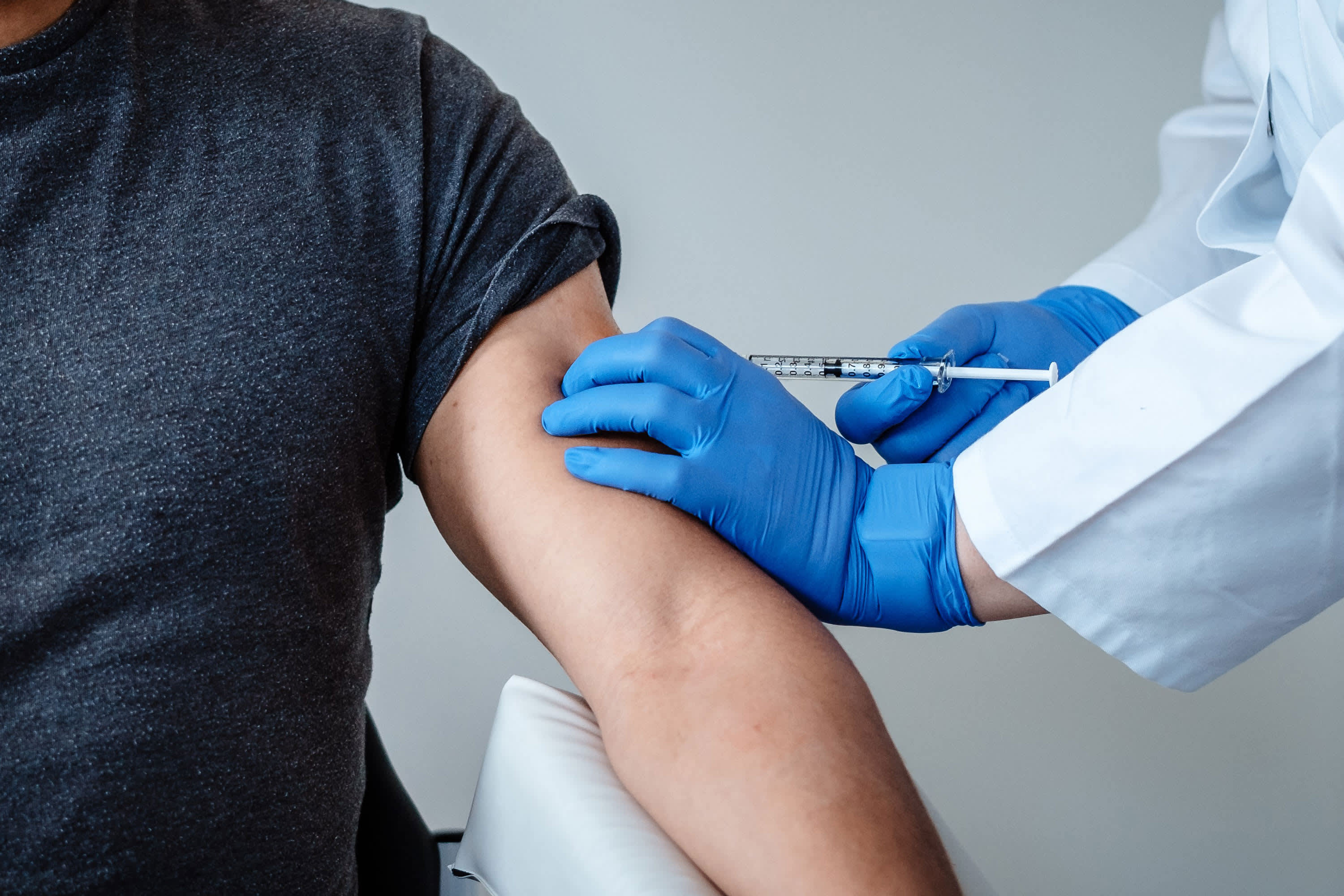
Komentar